By Avindra Nath and B. Jeanne Billioux
The crisis we are currently facing is unprecedented in every way. Just a few months ago, we were talking about developing targeted gene therapies for a spectrum of diseases, including ultrarare diseases. Only a few weeks later, the health care system finds itself overburdened and undersupplied to the point where we are talking about rationing health care1. Maintenance care has been pushed to telemedicine clinics and elective procedures have ground to a halt. Many patients sick with respiratory symptoms are being sent home to isolate themselves, and some are dying at home. There is an acute shortage of ventilators to the point that in some hospitals one ventilator is being shared by multiple patients2.
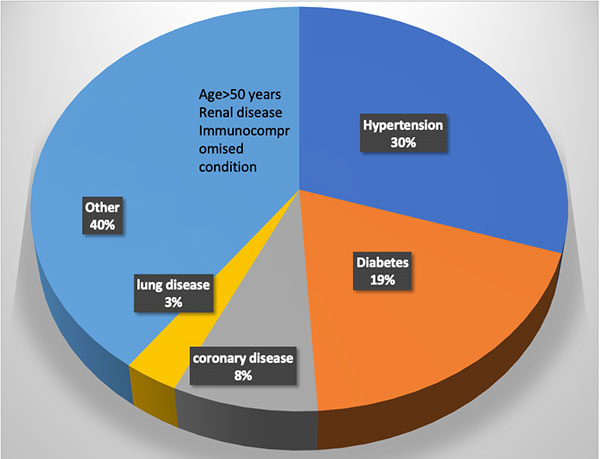
Figure 1. Distribution of comorbidities in patients requiring inpatient care due to COVID-19.
Several basic medicines are in limited supply. Although many hospitals and institutions recognized the need to stockpile personal protective equipment, several hospitals have run out of masks and gowns due to a limited supply chain. This crisis has tested community-based ingenuity, and in some hospitals, personal protective equipment is being fashioned by staff and community volunteers out of plastic visors and trash bags. Many doctors on the front line have succumbed to the infection, and many others are quarantined, a sobering reminder of these dire circumstances. The words here just a few months prior would read as a work of fiction, but this is the unfortunate reality of the crisis we face – COVID-19. Nearly every country and every major city in the world has been affected by the infection. On April 12 alone, there were over 10,000 new infections and nearly 1,000 deaths in a single day in New York. What started in Wuhan in November 2019 has become a global pandemic necessitating drastic changes in our way of life.
About COVID-19
COVID-19 is caused by the virus SARS-CoV2, a single-stranded RNA virus. Merely 60 nm in size, the virus that can only be visualized by an electron microscope has caused massive devastation. Although many pandemics have occurred in the past several decades, SARS-CoV2 has an array of features that have made it incredibly effective in spreading through the population. Perhaps the most important among these features is that asymptomatic and pre-symptomatic hosts can spread it. These asymptomatic carriers can infect large populations without knowing that they are infected with the virus. In fact, every time we speak, we release droplets into the air that can carry the virus a few feet.3 This property is the reason that distancing of at least six feet from one another and use of masks even made of cloth by the general public can be effective at lowering the degree of spread. Evidence suggests the virus can be easily inactivated by a wide variety of cleansing and disinfecting agents, including proper use of soap and water4.
Since the virus is spread through the respiratory passages, it manifests predominantly with respiratory symptoms. Most patients develop fever, dry cough, and fatigue/malaise, with many also reporting headache, myalgias, rhinorrhea, and anosmia with ageusia. Gastrointestinal symptoms occur in some patients. The symptoms may last for one to two weeks with nearly full recovery. Some symptoms such as fatigue may take longer to recover. However, nearly 20% to 30% patients may develop much more severe pulmonary symptoms. Toward the end of the first or second week, when other symptoms are improving, these patients develop dyspnea due to massive inflammation in the lungs caused by a viral pneumonia resulting in an acute respiratory distress syndrome. These patients require ventilatory support, and mortality rates are high. However, those patients who manage to survive the ordeal can recover with few residual symptoms, although the long-term consequences of the pulmonary damage are currently not known5. Patients who require hospitalization shed virus for an average of 20 days (range 8 to 37 days) from the time of symptom onset. The possibility that the virus may get reactivated has been raised. The Korean CDC is following 51 such patients who were thought to be cured but became positive again after leaving quarantine. If the virus is capable of reactivation, and whether reactivated virus is capable of infection, remains an open question. However, the findings in the Korean patients are likely related to false negative PCR testing.
Complications and Risk Factors
Multiple systemic complications may occur in patients who have severe respiratory symptoms. This may include myocarditis, which can be fatal in nearly 50% of those who develop it. A coagulopathy may occur in others resulting in both venous and/or arterial occlusions. Renal failure is a late complication of the disease.
Several risk factors have been identified for the severe manifestations of the illness. (See Figure 1). Interestingly, children only develop a mild illness and generally recover fully. Older adults have the highest risk. The complications seem to be more common in males. Hypertension and diabetes are also major risk factors which account for nearly 50% of the comorbidities in hospitalized patients6. The reasons for this are not entirely clear. One hypothesis suggests that since angiotensin converting enzyme 2 (ACE2) is the receptor for SARS-CoV-2, the use of ACE inhibitors to treat hypertension or diabetes can induce the expression of the receptor making the cells more vulnerable to infection with the virus. Clinical studies are underway to test this hypothesis. Current recommendations are to keep patients who are already on ACE inhibitors and ACE receptor blockers on their medications, as the risk of adverse events of discontinuing these medications may outweigh the minimization of risk for COVID-19.
As neurologists, we worry about our patients who have a chronic neurological illness. Can the illness itself or the medications that they are on put our patients at greater risk of severe illness? These questions are particularly important in the context of nursing homes, where neurologic comorbidities are common, and the virus has displayed rapid spread. Most certainly, patients with diseases such as Parkinson’s disease, stroke, myasthenia gravis, or other diseases that can impair mobility may also impair lung function. Patients with immune-mediated disorders such as multiple sclerosis, neuromyelitis optica, and myasthenia gravis who are on immunosuppressant drugs may be at risk for more severe complications of the illness. Various organizations such the National Multiple Sclerosis Society are collecting data on patients who develop COVID-19. These data repositories are going to be helpful in determining what medications pose greater risk of complications from the infection. In the meantime, recommendations and guidelines are emerging from various societies based on our current knowledge for the management of patients with stroke7, multiple sclerosis (nationalmssociety.org), epilepsy (ilae.org) and myasthenia gravis8.
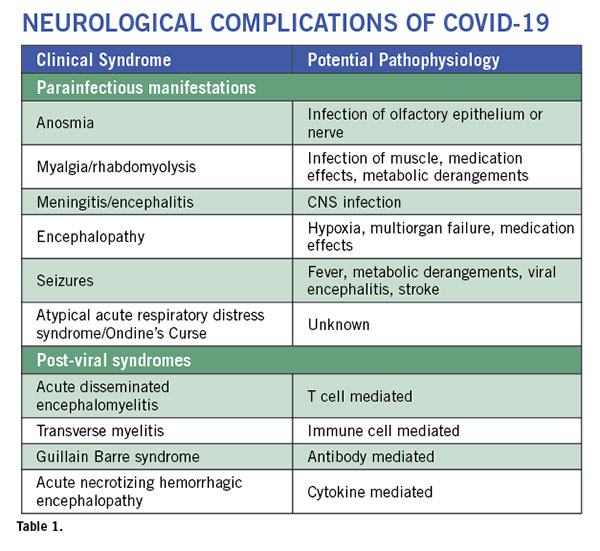
Neurological complications are rare but are being increasingly recognized9. These complications can involve the entire neuro-axis. They may occur during active viral infection and as a post-viral syndrome. (See Table 1). Some patients may present with altered mental status in the absence of respiratory or other typical COVID-19 symptoms as their sole initial presenting feature of SARS-CoV2 infection10. Anosmia is a common symptom of any upper respiratory tract infection. But anosmia with COVID-19 has received special attention. It seems to be one of the most common symptoms and often occurs in the absence of rhinorrhea. This suggests involvement of the olfactory nerve or pathway by the virus. As the majority of patients with anosmia recover their sense of smell and taste after the acute phase of the illness, the nerve endings or the cells surrounding the nerves may be affected, allowing for regeneration to occur. In a case report of a patient with sudden anosmia due to COVID-19, it was found that the olfactory clefts were inflamed, with relative sparing of the olfactory bulb11. In a mouse model of coronavirus infection, the virus can be transmitted via olfactory pathways trans-synaptically to the brain and to the brainstem12. This has raised concern about the potential long term consequences of anosmia in COVID-19. However, the mouse coronavirus uses a different receptor and hence may not replicate the human disease. Nevertheless, it is important to prospectively monitor the patients to make sure they do not develop any long-term sequelae since we know that anosmia is a recognized early symptom of neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease.
Strokes with COVID-19
Presentation
Venous sinus thrombosis
Ischemic strokes in multiple arterial distributions
Small blood vessel occlusions
Watershed Infarcts
Pathophysiology
Coagulopathy: elevated D-dimer, PT, aPTT
Antiphospholipid antibodies
Cardioembolic
Hypoperfusion
Risk factors
Myocarditis
Known vascular risk factors
ARDS and multiorgan impairment
Myalgia frequently accompanies the illness. Most viral illnesses can cause body aches and pains. However, in some patients with COVID-19, the muscle aches can be quite severe. Muscle tenderness may last for several days after all other symptoms have resolved. They can involve the back muscles. A case of rhabdomyolysis13 was reported similar to what was also seen with the SARS14, although this patient was also on lopinavir/ritonavir which may have contributed to the myolysis. Since the onset of these symptoms is early in the course of the illness, it is possible that the virus invades the muscle to cause myositis, however, pathological findings have not yet been described. Importantly, these patients need proper hydration to prevent kidney damage. Also, it should be noted that potential medications used in the treatment of COVID-19 (including some protease inhibitors) may cause patients to be predisposed to muscle damage.
Meningitis and encephalitis are rare. Dull headaches are common and typically occur at the onset of the illness and resolve within a few days. They are not accompanied by any signs of meningeal irritation. However, a classical presentation of a viral meningitis has been described with COVID-19 and virus can be detected in the CSF. Encephalitis is harder to diagnose. Most patients who become comatose do so after development of ARDS and multi-organ failure, hence the CNS symptoms are attributed to hypoxia and metabolic abnormalities. Fever itself can cause delirium. However, a few cases of encephalitis where patients developed generalized seizure and coma are now being described. In one such patient from Japan, the patient had mild pleocytosis and detectable virus in the CSF. An MRI showed lesions in the temporal lobe and adjacent ventriculitis15. Few neuropathological findings have been published, but one study found low levels of SARS-CoV-2 RNA in the brain by PCR of 4 different COVID-19 patients at autopsy15a. Another case study found evidence of betacoronaviral infection of the brain with postmortem electron microscopic evaluation15b. From the earlier SARS epidemic in 2003, autopsy findings showed that the virus could be detected in the brain by multiple techniques in all patients evaluated (n=8)16. Spread of SARS-CoV-2 into the brain could involve an array of mechanisms. The virus can spread via the vasculature and enter the brain carried by infected leukocytes. Transneuronal spread has been hypothesized to also occur from the lung via the vagal nerve or from the nasal passages via the olfactory nerve.
Strokes are being increasingly recognized in this population and occur as the presenting symptom of the infection or any time during the illness. (See Table 2.) In a study from China, 5% (n=11) of 211 patients admitted with COVID-19 had acute ischemic strokes, 0.5% (n=1) had cerebral venous thrombosis, and 0.5% (n=1) had cerebral hemorrhage17. While most often these patients have underlying vascular risk factors, there are several patients where nothing other than the SARS-CoV-2 infection can be identified as a cause of the stroke. The virus is known to invade endothelial cells and can also cause a coagulopathy. Elevated D-dimer levels and increased PT and activated PTT have been described. Antiphospholipid antibodies have also been detected18. Some may develop disseminated intravascular coagulation. The virus can also cause a cardiac myositis19 which could also cause a stroke by hypoperfusion or embolism. Some patients may simultaneously develop deep vein thrombosis or vascular occlusions in other organs.
Atypical Acute Respiratory Distress Syndrome is the major cause of death in patients with COVID-19. What is atypical is that these patients have severe hypoxemia even when the lung capacity and mechanics are well preserved20. Even when the pCO2 is rising the patients are not hyperventilating and lose their respiratory drive. They develop what seems like an Ondine’s Curse. However, these patients do not have any other brainstem signs so the pathophysiology of this condition remains unclear at the present time. However, it is critical that these patients be treated with oxygen, and prone positioning also seems to help. Early ventilatory support should also be considered.
Post-viral syndromes occur when the patient is seemingly improving from the viral syndrome at about a week to three weeks after the onset of the viral prodrome. An isolated case of acute necrotizing hemorrhagic encephalopathy has been described21. This patient had bilateral thalamic lesions and other lesions in the temporal lobes which are typical of the syndrome. It is thought to be mediated by cytokine storm. A patient with transverse myelitis with quadriparesis, a sensory level and bowel and bladder involvement has been described22. However, MRI or CSF evaluation was not reported. A single case of Guillain Barre Syndrome (GBS) has been published in a patient from China23. A case series from Italy of five COVID-19 patients who developed GBS described three patients with an axonal form of GBS and two with a demyelinating process24. We recently communicated with a patient who had a sensory variant of GBS. The illness was self-limiting and did not require intervention. Acute disseminated encephalomyelitis has been recently described in an adult patient with SARS-CoV-224a; similarly, several cases have been described with the human coronavirus-OC4325 and with MERS26.
However, multiple challenges in the evaluation of patients with neurological complications exist. It is difficult to get neuroimaging when patients are acutely infected for fear of contamination of the scanners. Performing surgery or autopsies are also challenging due to the production of aerosols and lack of proper safety measures.
Therapeutic Debate
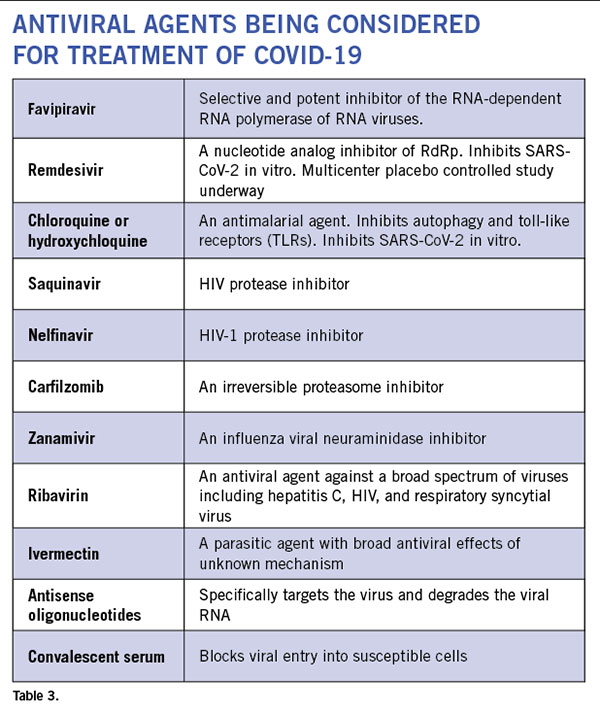
Anti-virals: Even though currently there is no proven antiviral therapy for the human coronaviruses, several drugs are being considered for clinical trials and empirical treatment of patients. (See Table 3.) There are 287 studies on coronavirus registered on www.clinicaltrials.gov. In vitro studies have shown some efficacy with chloroquine and hydroxychloroquine. These drugs cause acidification of the endosome-lysosomes and prevent viral replication. They have an anti-inflammatory effect. However, it requires pretreatment of cells prior to infection and has only a minimal effect post infection. While clinical trial results remain unpublished, these drugs have been utilized in clinic off-label in COVID-19 patients at several institutions. Well-controlled studies are necessary to know whether these drugs are efficacious against the virus. There is now a scarcity of the drug, and some countries have banned its export. Several HIV protease inhibitors have been shown to bind to the SARS-CoV-2 protease but clinical experience in small numbers of humans infected with the virus have failed to show clinical efficacy with lopinavir/ritonavir combination. Many clinical trials are currently underway that include nucleoside analogs such as remdesivir, and convalescent serum or intravenous immunoglobulin. Although the ability of most of these agents to enter the CNS is unknown, animal studies of remdesivir (GS-5734) have shown evidence of CNS penetrance, albeit at lower levels than other tissues27. Interestingly, a few drugs used to treat patients with multiple sclerosis such as teriflunomide and beta-interferons are considered to have anti-viral effects. But their effect on SARS-CoV-2 is still unknown.
Anti-inflammatory drugs: The most common cause of death is the massive immune response in the lungs leading to consolidation of the lungs with inflammatory infiltrates. Several immune suppressive medications are being used empirically. These include corticosteroids and IL-6 blockers. A case for the use of methotrexate has been made due to its broad anti-inflammatory properties and good CNS penetration.
What the Future Holds
As we continue to face the ongoing crisis, early results show reasons for optimism. In several states in the U.S., exponential growth trends have tapered. Distancing and preventive measures seem to be effective in flattening of the curve and helping institutions lower concomitant caseloads. The number of new infections and deaths are not rising as rapidly. Optimistically, we will soon face a new challenge of when and how to reopen our clinics and operating rooms. But what will this clinical environment look like? Social distancing is likely to play a role, and providers may see patients and enter any public spaces with masks on and maintain a distance of six feet from each other. Telemedicine will likely continue to play a much larger role in routine health care. A safe and effective vaccine could solve many of these issues, though development and testing of such a vaccine prior to administration to the general populace will take significant time. Another possibility is host adaptation. Most viruses are cyclical in nature. Mutations may occur that make the virus less virulent. Early signals suggest this might be the case with SARS-CoV-2. A 382 nucleotide deletion in open reading frame 8 has been identified in some circulating strains. A similar deletion also emerged in the SARS virus in 2003 that was associated with poor replication fitness28. However, until then, we will continue to see patients with COVID-19, and as neurologists we need to be vigilant for potential complications that require our attention and intervention. It is our duty to protect and advocate for the most vulnerable. •
Avindra Nath, MD, is chief of the Section of Infections of the Nervous System and Clinical Director, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health in Bethesda, Maryland.
B. Jeanne Billioux, MD, is staff clinician and head of the program in International Neuroinfectious Diseases within NINDS. Her research focus is on emerging infectious diseases and conducting research on the neurological consequences of infections in an International setting.
Correspondence
Avindra Nath, MD; Room 7C-103; Bldg. 10;
10 Center Drive, Bethesda, MD, 20982; U.S.
301-496-1561; e-mail: natha@ninds.nih.gov
References
1. Emanuel EJ, Persad G, Upshur R, et al. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. N Engl J Med 2020.
2. Truog RD, Mitchell C, Daley GQ. The Toughest Triage – Allocating Ventilators in a Pandemic. N Engl J Med 2020.
3. Lewis D. Is the coronavirus airborne? Experts can’t agree. Nature 2020;580:175.
4. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 2020;104:246-251.
5. Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020.
6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-1062.
7. Leadership AASC. Temporary Emergency Guidance to US Stroke Centers During the COVID-19 Pandemic. Stroke 2020.
8. International MGC-WG, Jacob S, Muppidi S, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci 2020;412:116803.
9. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 2020.
10. Filatov A SP, Hindi F, et al. . Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy. Cureus 2020;12:e7352. doi:7310.7759/cureus.7352.
11. Eliezer M, Hautefort C, Hamel AL, et al. Sudden and Complete Olfactory Loss Function as a Possible Symptom of COVID-19. JAMA Otolaryngol Head Neck Surg 2020.
12. Dube M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ. Axonal Transport Enables Neuron-to-Neuron Propagation of Human Coronavirus OC43. J Virol 2018;92.
13. Jin M, Tong Q. Rhabdomyolysis as Potential Late Complication Associated with COVID-19. Emerg Infect Dis 2020; 26.
14. Tsai LK, Hsieh ST, Chang YC. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwan 2005;14:113-119.
15. Moriguchi T, Harii N, Goto J, et al. A first Case of Meningitis/Encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis 2020.
15a. Menter, JD; Haslbauer, R; Nienhold, S, et. al. Post-morten examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 May 4.
15b. Paniz-Mondolfi A, Bryce C, Grimes Z, et al. Central Nervous System Involvement by Severe Acute Respiratory Syndrome Coronavirus -2 (SARS-CoV-2). J Med Virol. 2020 Apr 21.
16. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med 2005;202:415-424.
17. Li YW, W.; Zhou, Y.; et al., . Acute Cerebrovascular Disease Following COVID-19: A Single Center, Retrospective, Observational Study. Lancet 2020:https://ssrn.com/abstract=3550025.
18. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med 2020.
19. Kim IC, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J 2020.
20. Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. Covid-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2020.
21. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI Features. Radiology 2020:201187.
22. Zhou KH, J.; Dai, D.; Feng, Y.; Liu, L.; Nie, S. Acute myelitis after SARS-CoV-2 infection: a case report. https://wwwmedrxivorg/content/101101/2020031620035105v1fullpdf 2020.
23. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 2020.
24. Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barre Syndrome Associated with SARS-CoV-2. N Engl J Med 2020.
24a. Zhang T, Rodricks MB, Hirsh E. COVID-19-Associated Acute Disseminated Encephalomyelitis: A Case Report. medRxiv 2020.04.16.20068148; doi: https://doi.org/10.1101/2020.04.16.20068148
25. Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics 2004;113:e73-76.
26. Arabi YM, Harthi A, Hussein J, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection 2015;43:495-501.
27. Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016;531:381-385.
28. Su YCF, Anderson, D. E., Barnaby, Y. E, et al.,. Discovery of a 382-nt deletion during the early evolution of SARS-CoV-2. Biorxiv 2020;
https://doi.org/10.1101/2020.03.11.987222.
