By Girish Modi and Viness Pillay
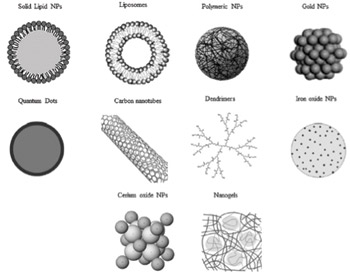
Figure 1. Different type of nanomaterials for biomedical use. Nanomaterials are commonly defined as objects with dimensions of 1-100 nm, which includes nanogels, nanofibers, nanotubes and nanoparticles (NP). In this illustration is represented the morphology of the most commonly used NP for therapy and diagnosis of neurodegenerative diseases (ND). (Ref. 3; reproduced with permission from Elsevier B.V. Ltd. © 2012.)
Nanotechnology employs engineered materials or devices (nanomaterials) with the smallest functional organization on the nanometer scale (1–100 nm) that are able to interact with biological systems at the molecular level1. They stimulate, respond to and interact with target sites to induce physiological responses while minimizing side effects1. By definition, nanomaterials are natural, incidental or manufactured materials containing particles (nanoparticles), in an unbound state or as an aggregate or as an agglomerate2. There are various methods of preparation of nanoparticles such as emulsion polymerization, interfacial –polymerization, –polycondensation, or –deposition, solvent –evaporation and –displacement, salting-out and emulsion/solvent diffusion, to name a few. There are several different types of nanostructures. These include polymeric nanoparticles, nanocapsules, nanospheres, nanogels, nanosuspensions, nanomicelles, nanoliposomes, carbon nanotubes and nanofibers3 (See Figure 1.)
Nanoparticulate matter and related materials possess an inherent capability to efficiently and effectively cross the blood brain barrier (BBB) leading to an increased concentration of encapsulated bioactives and drugs in the cerebrospinal fluid (CSF). In addition to drug delivery, this BBB crossing ability of the nanoparticles can be employed to develop various real-time diagnostic tools.
Promising features of nanosystems in targeted CNS drug delivery are that
- their chemical properties can be easily modified to achieve organ-, tissue-, or cell-specific and selective drug delivery
- the delivery of the drugs can be controlled
- they increase the bioavailability and efficacy of incorporated drugs by masking the physicochemical characteristics and thus increase the transfer of drug across the BBB
- they protect incorporated drugs against enzymatic degradation
- they have fewer side effects.1
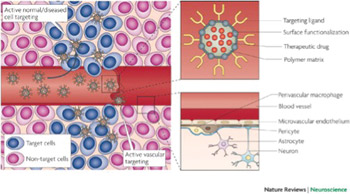
Figure 2. The neurovascular unit (bottom right panel) regulates the dynamic and continuous crosstalk between circulating blood elements and brain cellular components, including perivascular macrophages, astrocytes and neurons. At the interface between blood and brain (the blood-brain barrier), endothelial cells and associated astrocytes are stitched together by structures called tight junctions. The blood-brain barrier hinders the delivery of many potentially important diagnostic and therapeutic drugs to the CNS. The barrier results from the selectivity of the tight junctions between endothelial cells in the blood vessels that restricts the passage of solutes. Astrocyte cell projections called astrocytic feet surround the endothelial cells of the blood-brain barrier, providing biochemical support to those cells. The pericytes shown in this figure are undifferentiated mesenchymal-like cells that also line and support these vessels contributing to the complex layering of cells forming the blood-brain barrier. Nanocarriers (top right panel) are materials that can be configured into several different shapes (tubes, spheres, particles, rods, etc.) and can be loaded with drugs for sustained delivery to specific sites that are targeted by coating the surface of the material with peptides. In this figure, the nanocarrier contains a therapeutic drug, a targeting peptide to increase the penetration of the drug into the brain tissue and a functionalized surface consisting of, for example, an antibody to target a specific antigen or a steric coating made of polyethylene glycol or dextrans. Active targeting (left panel) enhances the biodistribution of drug-loaded nanocarriers in the brain tissue, improving the therapeutic efficacy of drugs. Once particles have extravasated in the target tissue, the presence of ligands on the particle surface facilitates their interaction with receptors that are present on target cells resulting in enhanced accumulation and cellular uptake through receptor-mediated processes (Ref. 4; reproduced with permission from Nature Publishing Group © 2009)
Taking these benefits into consideration, nanotechnology has immense potential such as in the field of neuromolecular diagnostics, discovery of neurodegenerative markers, nano-enabled drug delivery and neuroactive discovery with implications reaching to the prevention, management and treatment of neurological disorders.
In terms of delivery across the BBB, two basic approaches, namely the molecular approach and the polymeric carrier approach can be applied. In the molecular approach, nanonization or ligand attachment of the drugs can be employed to target brain cells, and the drugs can further be enzymatically activated afterward inside the target cell4. However, the availability of such modifiable drugs is low, and only certain drugs with specific functionality can be targeted molecularly. Additionally, a metabolic pathway is always required to activate these drugs inside CNS — further narrowing the options. The second approach of employing polymeric carriers such as drug-loaded nanoparticles can be termed as a universal approach with flexibility of choosing the matrix or polymer system and additionally can be administered by the route of choice: intravenously, intrathecally or as an implantable cerebral device. (See Figure 2.)
In this way, the BBB can be circumvented via systemic administration or CNS implantation with an ability to control as well as target the release of various bioactive agents used in the treatment of neurodegenerative disorders (NDs). The majority of nanotechnological drug delivery systems for the treatment of NDs are in the form of polymeric nanoparticles. Polymeric nanoparticles are promising for the treatment of NDs as they can pass through tight cell junctions, cross the BBB, achieve a high drug-loading capacity, and be targeted toward the mutagenic proteins.
Specific nanosystems explored for advanced experimental treatment of Alzheimer’s disease (AD), Parkinson’s disease and other CNS disorders are listed in Table 1.
Nano-enabled systems in the form of biodegradable and non-biodegradable templates for regeneration of damaged neurons and peptide-based self-assembling molecules have been employed as scaffolds for tissue engineering, neuroregeneration, neuroprotection and photolithography etching5. Nanoparticulate strategies in the form of bioactive nanoparticles are being employed to provide resealing, repair, regeneration, restoration and reorganization of neural tissue after traumatic spinal cord injury. Nanoparticles are capable of achieving this via the control of secondary injury cascade, reassembly of the tethered membranous structures, creating a neuropermissive microenvironment, rebooting the neuropathophysiologica; connections, and recovery of the sensorimotor responses6,7 (See Figure 3.) Silva and co-workers, 2004, reported the potential of self-assembling peptide nanofibers (SAPN) in neural tissue engineering wherein the nanofibers provided the “neurite-promoting laminin epitope IKVAV,” thereby inducing a rapid differentiation of cells into neurons via the amplification of bioactive epitope presentation and further restricting the development of astrocytes8.
The current therapeutic paradigms (using conventional drug delivery systems) employed to provide functional recovery in NDs lack adequate cyto-architecture restoration and connection patterns required to overcome the restrictive blood-brain barrier. In addition to the restrictive blood brain barrier; the neuroactive drug therapies present various other challenges such as:
- higher doses are required to provide significant therapeutic benefit
- low bioavailability further aggravating the first condition
- poor absorption even after systemic delivery
- the unwanted severe side-effects due to preferential uptake of drugs by peripheral cells.
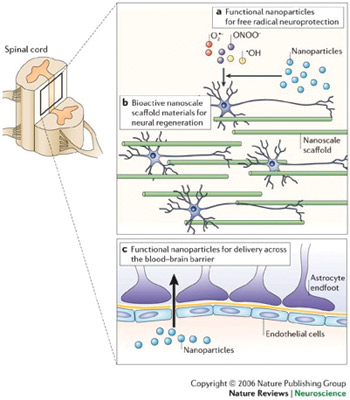
Applications of nanotechnology in clinical neuroscience. Nanotechnology can be used to limit and/or reverse neuropathological disease processes at a molecular level or facilitate and support other approaches with this goal. (a) Nanoparticles that promote neuroprotection by limiting the effects of free radicals produced following trauma (for example, those produced by CNS secondary injury mechanisms). (b) The development and use of nanoengineered scaffold materials that mimic the extracellular matrix and provide a physical and/or bioactive environment for neural regeneration. (c) Nanoparticles designed to allow the transport of drugs and small molecules across the blood-brain barrier (Ref. 7; reproduced with permission from Nature Publishing Group © 2006.)
The introduction and application of nanotechnological strategies may help in overcoming and even completely removing these neurotherapeutic challenges5.
Although various studies have claimed and demonstrated the potential of nanoparticles for CNS targeting, drug release and even gene delivery, rapidly biodegradable poly(butylcyanoacrylate) (PBCA) nanoparticulate system is the only successful nano-based drug delivery system that is being employed for the in vivo administration of drugs targeted to the brain. However, the mechanism of performance of this successful system has not been confirmed yet with three different reports stating entirely different mechanisms:
- polysorbate 80 coated PBCA nanoparticles reportedly cross the BBB via a carrier based approach by plasma adsorption of apolipoproteins resulting in receptor-mediated endocytosis by brain capillary endothelial cells9
- Kreuter and co-workers, 2002, suggested phagocytosis or endocytosis by endothelial cells as the possible transport mechanism through the BBB10
- Vauthier and co-workers, 2003, suggested that nanoparticle adhere to the cell membrane with subsequent escape by the P-glycoprotein efflux system to reach the CNS11.
These contrasting reports may lead to delay in regulatory approval of these promising nanosystems, and hence, further research into the exact elucidation of the mechanism of nanoparticle transport across the BBB is required.
Nanoscale classes of neuroactives will widen the scope of therapeutic action beyond merely modifying transmitter function to include stem cell and gene therapies that could offer a more selective mode of targeting. Nanoresearch focused on the regeneration and neuroprotection of the CNS will significantly benefit from the parallel advances in neurophysiology and neuropathology research. Therefore, for nanotechnology applications in neurology and neurosurgery to come to fruition, the following need to be considered:
- advancements in pharmaceutical chemistry and materials science that produce sophisticated synthetic and characterized approaches
- advancements in molecular biology, neurophysiology and neuropathology of the nervous system
- the design and integration of specific nano-enabled applications to the CNS which take advantage of the first two points.
- If these areas are developed in an integrated and parallel manner, nanotechnology based applications for NDs may begin to reach the clinic. As with all therapeutic approaches, for the treatment of CNS disorders, the challenge of targeting the material, device or drug to the site where it is needed always remains. Therefore, in order for nanotechnology applications directed toward NDs to be fully exploited, it would be
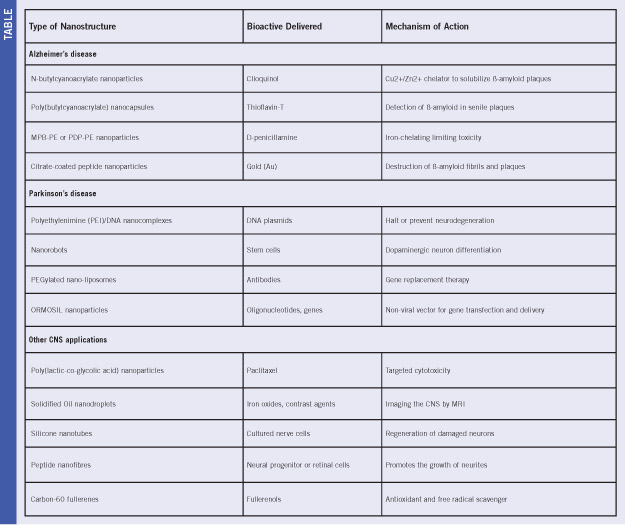
Table 1. Overview of various nano-enabled neurotherapies and interventions for CNS disorders (Ref. 5; reproduced with permission from Elsevier B.V. Ltd. © 2009.)
important for neurosurgeons, neurologists and neuroscientists to contribute to the scientific process along with pharmaceutical scientists and engineers. True to the highly interdisciplinary nature of this area of research, it is important that technological advancements occur in conjunction with basic and clinical neuroscience advancements5.
Modi is professor and head of Neurology, and the academic head of clinical neurosciences, Faculty of Health Sciences, University of the Witwatersrand. Pillay is professor of Pharmaceutics, Faculty of Health Sciences, University of the Witwatersrand.
References
- Modi G, Pillay V, Choonara YE. Advances in the treatment of neurodegenerative disorders employing nanotechnology. Ann NY Acad Sci. 2010;1184:154–172.
- Nanomaterials. http://ec.europa.eu/environment/chemicals/nanotech/faq/definition_en.htm (accessed on 20 November, 2013)
- Re F, Gregori M, Masserini M. Nanotechnology for neurodegenerative disorders. Nanomedicine: NBMedicine 2012;8:S51–S58.
- Orive G, Anitua E, Pedraz JL, Emerich DF. Biomaterials for promoting brain protection, repair and regeneration. Nat Rev Neurosci. 2009;10:682-692.
- Modi G, Pillay V, Choonara YE, Ndesendo VMK, du Toit LC, Naidoo D. Nanotechnological applications for the treatment of neurodegenerative disorders. Prog Neurobiol. 2009;88:272–285.
- Kumar P, Choonara YE, Modi G, Naidoo D, Pillay V. Nanoparticulate strategies for the 5 R’s of traumatic spinal cord injury intervention: restriction, repair, regeneration, restoration and reorganization. Nanomedicine, Manuscript accepted, In press, To be published in Feb 2014.
- Silva GA. Neuroscience nanotechnology: progress, opportunities and challenges. Nat Rev Neurosci. 2006;7:65-74.
- Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high–epitope density nanofibers. Science, 2004;303:1352-1355
- Kreuter J, Ramge P, Petrov V, Hamm S, Gelperina SE, Engelhardt B, Alyautdin R, von Briesen H, Begley DJ. Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm Res. 2003;20: 409–416.
- Kreuter J, Shamenkov D, Petrov V, Ramge P, Koch-Brandt C. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood–brain barrier. J Drug Target. 2002;10:317–325.
- Vauthier C, Dubernet C, Chauvierre C, Brigger I, Couvreur P. Drug delivery to resistant tumors: the potential of poly(alkyl cyanoacrylate) nanoparticles. J Control Release 2003;93:151–160.
