A preview of upcoming WFN projects and goals this year.
By Prof. Wolfgang Grisold
Welcome to the new edition of World Neurology, the newsletter of the World Federation of Neurology (WFN). Since the last issue, several projects have been announced and are forthcoming.
The deadline for applications for WFN trustee positions ended Feb. 14, 2025, and the Nominating Committee has listed the candidates for the positions of president, vice president, and one trustee. The list of nominees, short biographies, and statements from the candidates can be found in this issue as well as on the WFN website.
According to the bylaws, additional candidates supported by any of the five member societies can apply for any position until one month before the election. The election will be held virtually. The date and deadline will be announced on the WFN website. We look forward to interested and motivated candidates who will work for the WFN in the future. Traditionally, the trustees are not involved in the process of recommendation.
Individuals with experience in the tasks of the WFN will be welcomed to apply, and their expertise is needed. Information on the work of the WFN can be found on the WFN website, the WFN essentials page, and the newest Journal of the Neurological Sciences (JNS) Service Page.
The main WFN activities can be summarized by two letters: A and T. A is for advocacy and global activities, and T is for teaching and training.
Advocacy

Dr. Tedros Adhanom Ghebreyesus (left), director general of the WHO at the Geneva meeting 2025, pleading for more attention for emergency activities.
Advocacy for neurology is a growing part of the WFN’s activities. The importance of advocacy is implemented in the World Health Organization’s (WHO) Intersectoral Global Action Plan (IGAP). It is the core of WFN’s activities to communicate and work with the WHO and the U.N. Economic and Social Council (ECOSOC).
We need to increase the teaching of advocacy at all levels of health services. In addition to the WFN patient forums, we have a joint project with the American Academy of Neurology (AAN), the Global Advocacy Leadership Program (GALP), which will be presented for the first time this year at the AAN Annual Meeting, the World Congress of Neurology (WCN), and virtually.
The WHO’s brain health initiatives and the IGAP are supported by the WFN. The WFN community of neurologists considers the IGAP a unique opportunity to implement neurology in countries in need, to encourage all countries to invest in research and innovation, and to engage more in public health.
The U.N. ECOSOC is well defined by the 17 Sustainable Development Goals (SDGs). In particular, SDG 3 (good health and well-being) and SDG 5 (gender equality) are important for the WFN. Health is a central, but not the only, component of the work of the U.N. ECOSOC. We are appreciative that the U.N. ECOSOC will support this year’s World Brain Day (WBD) on brain health.
The GALP is a unique project of the WFN and AAN to increase awareness of the need for advocacy and to teach both advocacy and leadership skills. The course has many elements of the successful AAN Palatucci Advocacy Leadership courses and will add several topics of global relevance.
Twenty people from low-middle and low-income countries were selected from 100 applicants and will be fully supported to attend. After a face-to-face meeting, which took place at the AAN Annual Meeting in April in San Diego, the participants will attend a series of five virtual meetings and a final meeting and graduation at the World Congress of Neurology in October in Seoul, South Korea. Also, both the AAN and the WFN will waive the congress fees, so that participants may attend both meetings.

Prof. El Alaoui (left) studies the recognition certificate for the Training Center in Rabat during the WFN site visit. Prof. Nahzda Birouk (center), incoming Training Center chair, looks on with Prof. Wolfgang Grisold, WFN president.
Teaching
The educational activities of the WFN have included a visit to all Training Centers in Africa. A visit to Mexico City is planned for this year. In addition to the numerous reports, the African Training Centers received an analysis summary of the site visits, and we hope to continue this important activity.
The financial burden of the Training Centers is almost entirely on the WFN, although we have help from the Association of British Neurologists (ABN) for the Training Center in Cairo, and our successful Specialty Group at the International Congress on Neuromuscular Diseases regularly supports a large number of trainees in Rabat on electrophysiologic and neuromuscular training.
In related news, Prof. Nahzda Birouk will take over responsibilities from Prof. El Alaoui as chair of the Training Center in Rabat. We thank Prof. El Alaoui for his longstanding support and merits from the WFN.

Watching the results from the EMG machine during the WFN site visit in Rabat.
There are three four-year trainees in Africa and five one-year fellowships in Africa (general neurology, neuromuscular, epilepsy, and stroke). In Mexico City, we have a one-year fellowship on stroke. This is a large number for the WFN, but only a small crystal to offer for the 1.4 billion inhabitants on the African and Central and South American continents. However, crystals grow at various speeds, from minutes to many years.
Over the last 10 years, the North African centers, along with Senegal and Cape Town, have trained additional people. Several African universities are also poised to take up neurology training. This emphasizes the need for, and the increasing efforts of, training in Africa for Africans. “Empower the regions” is not just a concept; it is producing powerful teaching instruments, which will have long and enduring effects.

Cynthia Marleny Aliñado Ramos, the present WFN trainee, at the Training Center in Mexico City.
The increase in the number of neurologists is hoped to be exponential. This is only the peak of a needs pyramid, requiring other health care professionals, structures such as labs, imaging, testing, and inpatient and outpatient facilities, and access for all in need.
Although trained neurologists are forming the top level of that pyramid, there is also the model of the inverted pyramid, which on its large base has the most frequent and most important content. Seeing and appreciating the large needs for neurology, our effort must be directed toward implementing neurological care and knowledge into primary care. Increasing awareness of the most frequent neurological symptoms and signs, as well as the most frequent neurological diseases, will be important.
Education Days

Figure 1: Word bubble from the participants of the AOAN Education Day with requests for future topics.
Education and teaching have been successfully achieved with the most recent Education Day, held jointly with WFN, the Asian and Oceanian Association of Neurology (AOAN), and the Movement Disorders Society (MDS). The quality of the course was excellent, and the topics were of worldwide importance. We had a record number of registrants, and more than 1,000 participants. We will continue to partner with AOAN on more Education Days in the future.
The past series of Education Days, which were held over several years with the International Headache Society (IHS) and the Global Patient Advocacy Coalition (GPAC) were also successful. The most recent African Education Day on neuropathies was held in 2024. The preparation, organization, and financing of these events is an important task of the WFN, which needs strong cooperating partners.
World Brain Day (WBD)
World Brain Day 2025 will feature the topic of brain health, which seems to be attracting attention worldwide. The full wording of this year’s theme is “Brain Health for All Ages.” This wording concurs with the United Nations SDG3 (good health and well-being) and is a fundamental part of healthy living. It will increase attention toward disease groups in different age groups and regions.
There is also a great divide between communicable and noncommunicable diseases (NCDs). Despite the global increase of NCDs, infections worldwide still present a challenge, which is often underestimated. The lessons from the COVID-19 pandemic concern all of us, and we can see that the WHO prepares and invests in emergency structures for the future.
World Congress of Neurology (WCN)

A successful Coffee Talk at the WCN 2023 in Montreal. Left to right: Ashley Logan (moderator), WFN President Prof. Wolfgang Grisold, with WFN past presidents Prof. Vladimir Hachinski, Prof. Bill Carroll, and Prof. Raad Shakir.
WCN 2025 will take place Oct. 12-15 in Seoul, South Korea. We are grateful to the Korean Neurological Association (KNA) and to the region for supporting this important showcase for neurology in Asia.
Asia is large in geography and population, and access to neurology varies in many countries. The WCN is not only intended to discuss developments and updates, but will also have a series of brain health topics, including plenary lectures, dedicated global lectures, and regional lectures. These topics are dedicated to global health, indicating the important role for neurologists to increase and add to their activities.
The WCN will also have five plenary lectures, with topics ranging from the importance of the WHO to advances in the concept of senescence. Several joint sessions are planned with world neurological societies such as the WHO, MDS, International League Against Epilepsy (ILAE), and the Peripheral Nerve Society (PNS).

A look at the future stage for Coffee Talks at the WCN 2025 in Seoul.
Much attention is dedicated to the Teaching Course program. Similar to the WCN in Montreal, we will expand our interactive programs, which will be a series of “coffee talks.” We will also feature a program on Continuum, and include young neurologists from the region. We will continue the concept of a hybrid meeting as the participation in the hybrid form was great, and we had a reach of 135 countries. Costs for hybrid attendance will be kept low to allow participants from low-middle and low-income countries to participate.
There will be social programs and opportunities to network and sightsee in Seoul.
Publications
It is the determination of the WFN to be heard in as many forms of publication as possible. The WFN website provides information on the structure and substance of the WFN. It is also used for news and information distribution. This is supported by our social media feeds, where the WFN teams post information.
World Neurology is the active newsletter, collecting articles worldwide. It is free to download and also offers access to the World Neurology archive. The official journal of the WFN is the Journal of the Neurological Sciences (JNS), with John England as the editor-in-chief, and the eNS with Walter Struhal as the editor-in-chief.
For the JNS, we are adding quarterly Service Pages, and the eNS now has 10 articles from the WFN Digital Update (WNU) course available as open access.
We are working on a collective book on neurology called the White Book of Neurology. It will be edited by Prof. Alla Guekht, Steven Lewis, Prof. Riadh Gouider, and myself, and will be published by Springer. The idea is to delineate and describe the present structure of neurology, from the historical development toward the future.
Monthly Google Analysis
The monthly Google analysis shows an increase in followers and interested colleagues and parties. We want to thank our constantly leading countries — the U.S., India, and Great Britain — for their interest. •












 Hosted in Montreal, the 2023 World Congress of Neurology united experts to address key neurology challenges. Plenary speakers shared their latest research on topics such as diabetic neuropathies, Parkinson’s disease, ALS, and global health care equity.
Hosted in Montreal, the 2023 World Congress of Neurology united experts to address key neurology challenges. Plenary speakers shared their latest research on topics such as diabetic neuropathies, Parkinson’s disease, ALS, and global health care equity.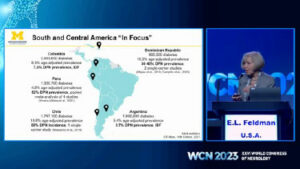


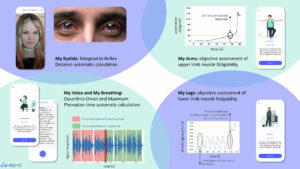
 One must first realize that more than three-quarters of the disease burden resides in regions that are struggling to bridge the treatment gap. In a world where everything is run by money, the catastrophic expenditure on health burdens every family in the lesser privileged sections of economy. This expenditure encompasses costs of medication, hospitalization, and continuation of care. The onus to balance livelihood and health falls on the patient, and when this burden becomes too much, they tend to stop being compliant to treatment — an ignition to the vicious cycle of expense and hospitalization.
One must first realize that more than three-quarters of the disease burden resides in regions that are struggling to bridge the treatment gap. In a world where everything is run by money, the catastrophic expenditure on health burdens every family in the lesser privileged sections of economy. This expenditure encompasses costs of medication, hospitalization, and continuation of care. The onus to balance livelihood and health falls on the patient, and when this burden becomes too much, they tend to stop being compliant to treatment — an ignition to the vicious cycle of expense and hospitalization.  Among the patient stories we have permission to share was of one college student on three anti-seizure medicines. He often received different injections in the emergency department and was prescribed different cocktails of medicines due to his lack of record keeping. But with this app, he is documenting his prescriptions and scans and has currently been seizure free for eight months. The most useful feature for him, “For me, the drug reminder alarm is a huge help, and it makes sure I have not missed medications like I used to before.”
Among the patient stories we have permission to share was of one college student on three anti-seizure medicines. He often received different injections in the emergency department and was prescribed different cocktails of medicines due to his lack of record keeping. But with this app, he is documenting his prescriptions and scans and has currently been seizure free for eight months. The most useful feature for him, “For me, the drug reminder alarm is a huge help, and it makes sure I have not missed medications like I used to before.”
 The five pillars of the World Health Organization’s (WHO)
The five pillars of the World Health Organization’s (WHO) 

